Recently, Wu Guozhong's research group of the Radiation Chemistry Laboratory, Shanghai Institute of Applied Physics, Chinese Academy of Sciences has made new progress in studying the phase behavior of ionic liquids in confined spaces, and related research results have been published in the Journal of Physical Chemistry Letters.
Ionic liquids are a type of special salts composed entirely of anions and cations. Due to their unique physical and chemical properties, such as almost non-volatile, non-flammable, and good thermal stability, ionic liquids are used in many fields such as synthesis, separation, and catalysis. In particular, it has a wide range of uses in surface catalysis, electrolytes in fuel cells and conductive composite materials, and the application will involve ionic liquids limited to nanospace, and its phase transition research is a hot spot in this field.
The study found that the ionic liquids in different atmospheres filled the pores of porous SiO2 (average pore diameter 4nm), and their physical and chemical properties showed huge differences. Under high vacuum conditions, the melting point of ionic liquid filled with SiO2 increased significantly, rising by about 140 degrees Celsius, which is different from the results reported in other literatures. Most literatures report that the melting point of ionic liquid filled in nanospace decreases. According to the analysis, filling the ionic liquid with high vacuum can make the ionic liquid fill almost the entire pore channel (as shown in the upper diagram of Figure 1). The system only has a solid-liquid interface, and the strong interface induction and nano-limiting effect at the nanometer scale lead to the ionic liquid Presenting crystal-like properties, the high-resolution TEM image also shows that the ionic liquid is evenly dispersed in the pores after filling (Figure 2c). When filling under normal pressure, due to the presence of compressed gas in the pores, the ionic liquid can only be adsorbed at the entrance of the pore or partially enter the interior of the pore. The system forms a complex gas-liquid-solid three-phase interface, and the molecular arrangement of the ionic liquid is relatively loose. , Causing the melting point of the ionic liquid to drop.
Paper information: Unravelling the Role of the Compressed Gas on Melting Point of Liquid Confined in Nanospace, J. Phys. Chem. Lett. 2012, 3: 1052−1055.
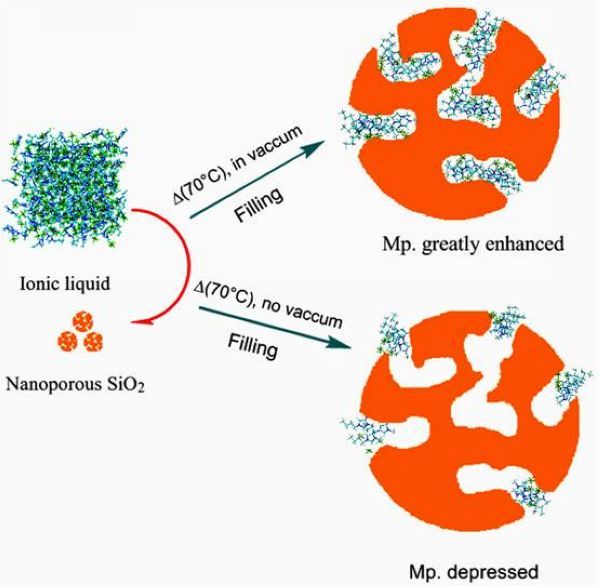 Figure 1. Schematic diagram of ionic liquid filling porous SiO2 in different atmospheres
Figure 1. Schematic diagram of ionic liquid filling porous SiO2 in different atmospheres 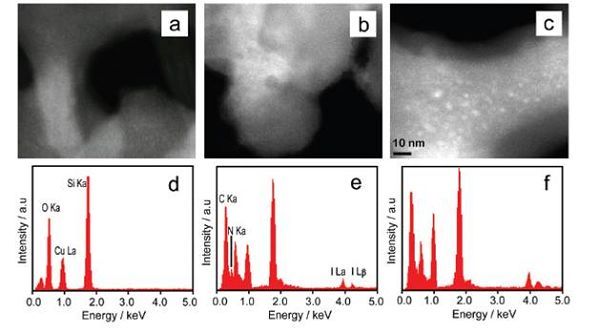 Figure 2. Comparison of high-resolution STEM graphs and EDX spectra: SiO2 (a, d), SiO2 (b, e) loaded with ionic liquid, SiO2 (c, f) filled with ionic liquid
Figure 2. Comparison of high-resolution STEM graphs and EDX spectra: SiO2 (a, d), SiO2 (b, e) loaded with ionic liquid, SiO2 (c, f) filled with ionic liquid
Face recognition: built-in database, which can perform a real-time comparison of captured faces locally, and generate a log of the comparison results and upload it to the background database
Access control: based on the results of face recognition, control the access switch to achieve personnel management in the controlled area
Body temperature detection: The device has a built-in body temperature monitoring module, which can simultaneously monitor human body temperature and obtain body temperature related data during face recognition.
face recognition temperature, infrared temperature instrument, body temperature scanner, infrared temperature instrument, thermal body temperature measuring
Guangzhou Sosu Electronic Technology Co., Ltd. , https://www.sosuchina.com